Which Two Substances Bind Using A Lock-and-key Mechanism?
Chapter 12. Kinetics
12.seven Catalysis
Learning Objectives
Past the finish of this section, you will be able to:
- Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams
- Listing examples of catalysis in natural and industrial processes
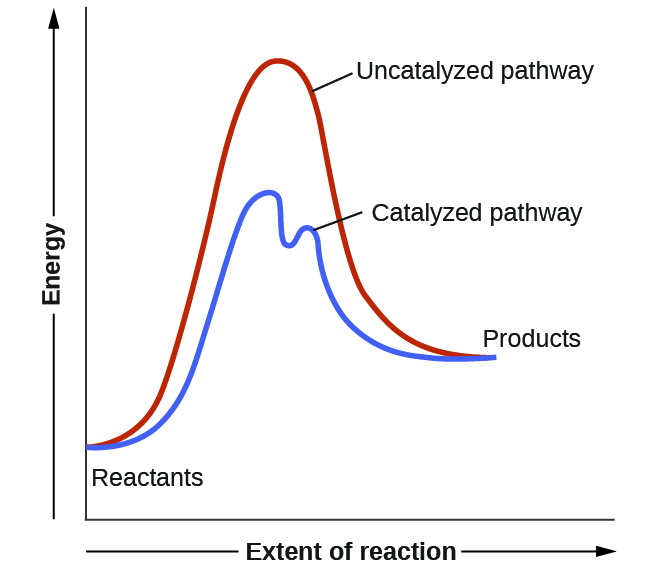
Nosotros have seen that the rate of many reactions can exist accelerated by catalysts. A goad speeds up the rate of a reaction by lowering the activation energy; in addition, the catalyst is regenerated in the process. Several reactions that are thermodynamically favorable in the absenteeism of a catalyst but occur at a reasonable rate when a catalyst is present. One such reaction is catalytic hydrogenation, the process by which hydrogen is added across an alkene C=C bond to beget the saturated alkane production. A comparison of the reaction coordinate diagrams (besides known equally energy diagrams) for catalyzed and uncatalyzed alkene hydrogenation is shown in Figure ane.

Catalysts role past providing an alternate reaction mechanism that has a lower activation free energy than would exist found in the absenteeism of the catalyst. In some cases, the catalyzed mechanism may include boosted steps, as depicted in the reaction diagrams shown in Figure 2. This lower activation energy results in an increase in rate as described by the Arrhenius equation. Notation that a catalyst decreases the activation free energy for both the forrad and the opposite reactions and hence accelerates both the forward and the contrary reactions. Consequently, the presence of a catalyst will allow a system to accomplish equilibrium more apace, but it has no effect on the position of the equilibrium as reflected in the value of its equilibrium abiding (see the later chapter on chemical equilibrium).
Example 1
Using Reaction Diagrams to Compare Catalyzed Reactions
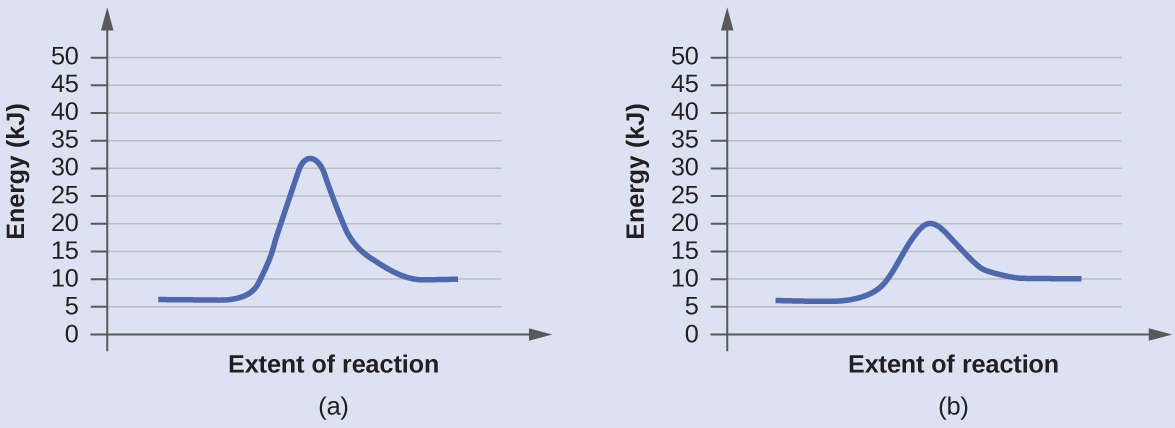
The two reaction diagrams hither represent the same reaction: 1 without a catalyst and one with a goad. Place which diagram suggests the presence of a goad, and make up one's mind the activation energy for the catalyzed reaction:
Solution
A catalyst does not affect the energy of reactant or product, so those aspects of the diagrams can be ignored; they are, as we would expect, identical in that respect. There is, however, a noticeable difference in the transition state, which is distinctly lower in diagram (b) than it is in (a). This indicates the apply of a catalyst in diagram (b). The activation free energy is the departure between the energy of the starting reagents and the transition state—a maximum on the reaction coordinate diagram. The reagents are at 6 kJ and the transition state is at twenty kJ, so the activation free energy can be calculated as follows:
[latex]E_{\text{a}} = 20\;\text{kJ}\;-\;six\;\text{kJ} = 14\;\text{kJ}[/latex]
Check Your Learning
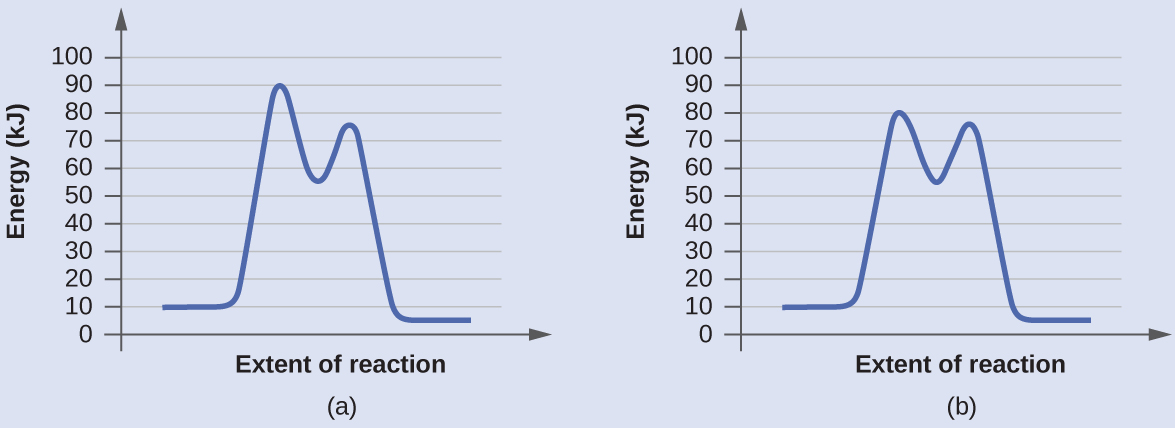
Determine which of the two diagrams here (both for the same reaction) involves a catalyst, and identify the activation energy for the catalyzed reaction:
Answer:
Diagram (b) is a catalyzed reaction with an activation energy of about seventy kJ.
Homogeneous Catalysts
A homogeneous goad is nowadays in the same phase as the reactants. It interacts with a reactant to form an intermediate substance, which then decomposes or reacts with another reactant in i or more steps to regenerate the original goad and form product.
As an of import illustration of homogeneous catalysis, consider the world's ozone layer. Ozone in the upper temper, which protects the earth from ultraviolet radiation, is formed when oxygen molecules absorb ultraviolet calorie-free and undergo the reaction:
[latex]3\text{O}_2(thou)\;{\xrightarrow{hv}}\;2\text{O}_3(thou)[/latex]
Ozone is a relatively unstable molecule that decomposes to yield diatomic oxygen by the opposite of this equation. This decomposition reaction is consistent with the following mechanism:
[latex]\brainstorm{array}{r @{{}\longrightarrow{}} l} \text{O}_3 & \text{O}_2\;+\;\text{O} \\[0.5em] \text{O}\;+\;\text{O}_3 & ii\text{O}_2 \end{array}[/latex]
The presence of nitric oxide, NO, influences the rate of decomposition of ozone. Nitric oxide acts as a catalyst in the following mechanism:
[latex]\begin{array}{r @{{}\longrightarrow{}} 50} \text{NO}(m)\;+\;\text{O}_3(g) & \text{NO}_2(g)\;+\;\text{O}_2(g) \\[0.5em] \text{O}_3(thou) & \text{O}_2(g)\;+\;\text{O}(k) \\[0.5em] \text{NO}_2(k)\;+\;\text{O}(g) & \text{NO}(g)\;+\;\text{O}_2(g) \end{assortment}[/latex]
The overall chemical change for the catalyzed machinery is the same as:
[latex]ii\text{O}_3(g)\;{\longrightarrow}\;three\text{O}_2(g)[/latex]
The nitric oxide reacts and is regenerated in these reactions. It is not permanently used up; thus, it acts equally a goad. The charge per unit of decomposition of ozone is greater in the presence of nitric oxide considering of the catalytic action of NO. Certain compounds that contain chlorine likewise catalyze the decomposition of ozone.
Mario J. Molina
The 1995 Nobel Prize in Chemistry was shared past Paul J. Crutzen, Mario J. Molina (Figure 3), and F. Sherwood Rowland "for their piece of work in atmospheric chemical science, particularly concerning the formation and decomposition of ozone."[i] Molina, a Mexican citizen, carried out the bulk of his piece of work at the Massachusetts Institute of Engineering (MIT).
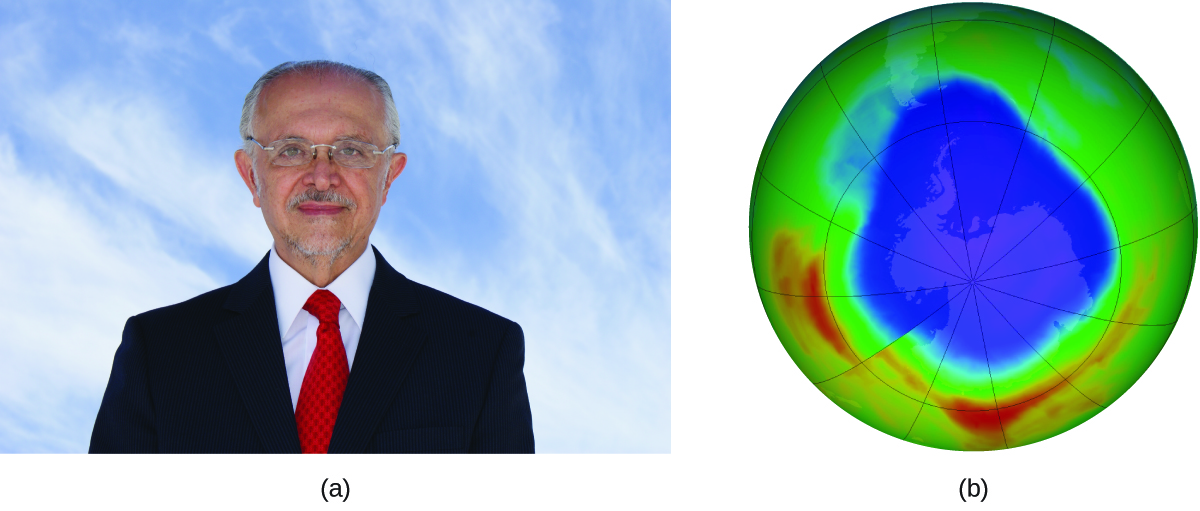
In 1974, Molina and Rowland published a paper in the periodical Nature (i of the major peer-reviewed publications in the field of scientific discipline) detailing the threat of chlorofluorocarbon gases to the stability of the ozone layer in world'south upper temper. The ozone layer protects earth from solar radiation by arresting ultraviolet light. As chemic reactions deplete the amount of ozone in the upper atmosphere, a measurable "pigsty" forms above Antarctica, and an increment in the amount of solar ultraviolet radiation— strongly linked to the prevalence of skin cancers—reaches earth'due south surface. The work of Molina and Rowland was instrumental in the adoption of the Montreal Protocol, an international treaty signed in 1987 that successfully began phasing out production of chemicals linked to ozone destruction.
Molina and Rowland demonstrated that chlorine atoms from human-made chemicals can catalyze ozone devastation in a procedure like to that by which NO accelerates the depletion of ozone. Chlorine atoms are generated when chlorocarbons or chlorofluorocarbons—once widely used as refrigerants and propellants—are photochemically decomposed past ultraviolet low-cal or react with hydroxyl radicals. A sample mechanism is shown hither using methyl chloride:
[latex]\text{CH}_3\text{Cl}\;+\;\text{OH}\;{\longrightarrow}\;\text{Cl}\;+\;\text{other\;products}[/latex]
Chlorine radicals break downwardly ozone and are regenerated past the post-obit catalytic cycle:
[latex]\begin{array}{r @{{}\longrightarrow{}} l} \text{Cl}\;+\;\text{O}_3 & \text{ClO}\;+\;\text{O}_2 \\[0.5em] \text{ClO}\;+\;\text{O} & \text{Cl}\;+\;\text{O}_2 \\[0.5em] \text{overall\;Reaction:\;O}_3\;+\;\text{O} & ii\text{O}_2 \end{array}[/latex]
A single monatomic chlorine tin can break downwardly thousands of ozone molecules. Luckily, the majority of atmospheric chlorine exists as the catalytically inactive forms Cltwo and ClONO2.
Since receiving his portion of the Nobel Prize, Molina has continued his work in atmospheric chemistry at MIT.
Glucose-six-Phosphate Dehydrogenase Deficiency
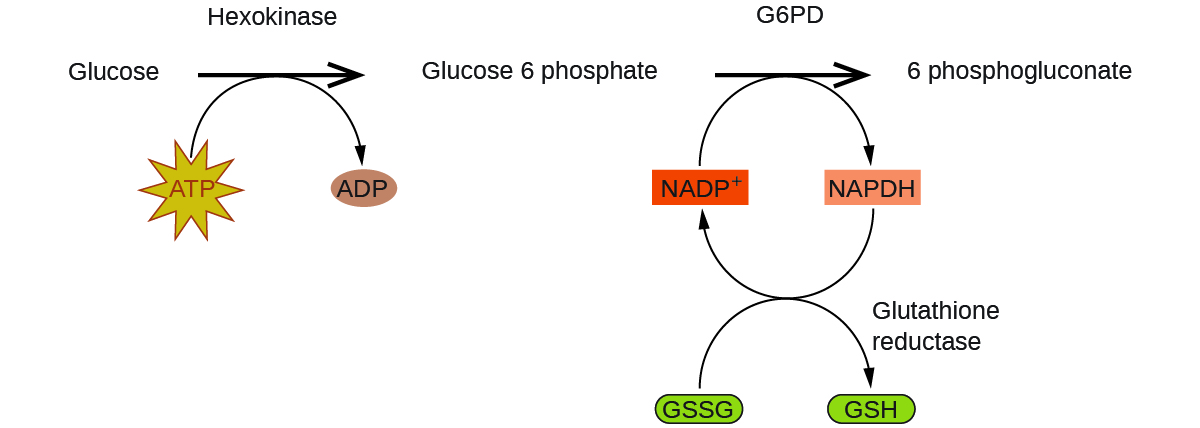
Enzymes in the human trunk act as catalysts for important chemic reactions in cellular metabolism. As such, a deficiency of a particular enzyme can translate to a life-threatening disease. G6PD (glucose-6-phosphate dehydrogenase) deficiency, a genetic status that results in a shortage of the enzyme glucose-6-phosphate dehydrogenase, is the most common enzyme deficiency in humans. This enzyme, shown in Effigy 4, is the charge per unit-limiting enzyme for the metabolic pathway that supplies NADPH to cells (Effigy v).
A disruption in this pathway can lead to reduced glutathione in ruby-red blood cells; once all glutathione is consumed, enzymes and other proteins such as hemoglobin are susceptible to damage. For example, hemoglobin can be metabolized to bilirubin, which leads to jaundice, a condition that can become severe. People who suffer from G6PD deficiency must avoid certain foods and medicines containing chemicals that can trigger harm their glutathione-scarce red blood cells.
Heterogeneous Catalysts
A heterogeneous catalyst is a catalyst that is nowadays in a different phase (usually a solid) than the reactants. Such catalysts by and large office past furnishing an active surface upon which a reaction tin can occur. Gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the goad rather than within the gas or liquid stage.
Heterogeneous catalysis has at least four steps:
- Adsorption of the reactant onto the surface of the catalyst
- Activation of the adsorbed reactant
- Reaction of the adsorbed reactant
- Improvidence of the product from the surface into the gas or liquid stage (desorption).
Any one of these steps may exist boring and thus may serve as the rate determining step. In general, however, in the presence of the catalyst, the overall charge per unit of the reaction is faster than it would exist if the reactants were in the gas or liquid phase.
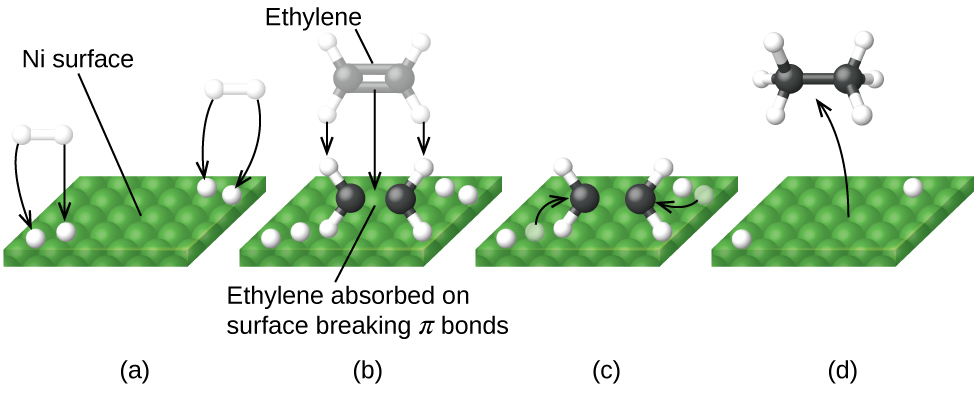
Figure six illustrates the steps that chemists believe to occur in the reaction of compounds containing a carbon–carbon double bail with hydrogen on a nickel catalyst. Nickel is the catalyst used in the hydrogenation of polyunsaturated fats and oils (which contain several carbon–carbon double bonds) to produce saturated fats and oils (which comprise only carbon–carbon single bonds).
Other significant industrial processes that involve the utilize of heterogeneous catalysts include the preparation of sulfuric acid, the preparation of ammonia, the oxidation of ammonia to nitric acid, and the synthesis of methanol, CH3OH. Heterogeneous catalysts are likewise used in the catalytic converters establish on most gasoline-powered automobiles (Figure vii).
Automobile Catalytic Converters
Scientists developed catalytic converters to reduce the amount of toxic emissions produced by called-for gasoline in internal combustion engines. Catalytic converters have advantage of all five factors that affect the speed of chemical reactions to ensure that frazzle emissions are equally rubber every bit possible.
By utilizing a carefully selected blend of catalytically active metals, it is possible to effect consummate combustion of all carbon-containing compounds to carbon dioxide while also reducing the output of nitrogen oxides. This is particularly impressive when nosotros consider that 1 step involves adding more oxygen to the molecule and the other involves removing the oxygen (Figure 7).

Most modern, 3-manner catalytic converters possess a surface impregnated with a platinum-rhodium catalyst, which catalyzes the conversion nitric oxide into dinitrogen and oxygen also as the conversion of carbon monoxide and hydrocarbons such as octane into carbon dioxide and water vapor:
[latex]\begin{assortment}{r @{{}\longrightarrow{}} 50} 2\text{NO}_2(thousand) & \text{N}_2(g)\;+\;2\text{O}_2(1000) \\[0.5em] 2\text{CO}(g)\;+\;\text{O}_2(one thousand) & ii\text{CO}_2(k) \\[0.5em] 2\text{C}_8\text{H}_{xviii}(yard)\;+\;25\text{O}_2(g) & 16\text{CO}_2(g)\;+\;18\text{H}_2\text{O}(1000) \end{array}[/latex]
In lodge to be as efficient as possible, most catalytic converters are preheated past an electric heater. This ensures that the metals in the catalyst are fully active even earlier the automobile exhaust is hot enough to maintain appropriate reaction temperatures.

The University of California at Davis' "ChemWiki" provides a thorough explanation of how catalytic converters piece of work.
Enzyme Structure and Role
The study of enzymes is an important interconnection between biology and chemistry. Enzymes are usually proteins (polypeptides) that help to command the rate of chemical reactions between biologically important compounds, especially those that are involved in cellular metabolism. Dissimilar classes of enzymes perform a variety of functions, every bit shown in Table 36.
| Class | Function |
|---|---|
| oxidoreductases | redox reactions |
| transferases | transfer of functional groups |
| hydrolases | hydrolysis reactions |
| lyases | grouping emptying to class double bonds |
| isomerases | isomerization |
| ligases | bond formation with ATP hydrolysis |
| Table 36. Classes of Enzymes and Their Functions | |
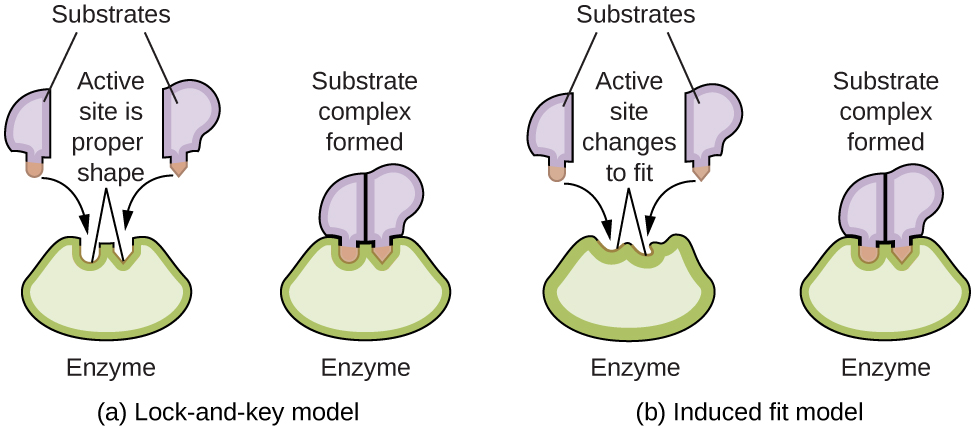
Enzyme molecules possess an active site, a part of the molecule with a shape that allows information technology to bond to a specific substrate (a reactant molecule), forming an enzyme-substrate circuitous every bit a reaction intermediate. There are ii models that attempt to explain how this active site works. The most simplistic model is referred to equally the lock-and-primal hypothesis, which suggests that the molecular shapes of the active site and substrate are complementary, plumbing fixtures together like a cardinal in a lock. The induced fit hypothesis, on the other manus, suggests that the enzyme molecule is flexible and changes shape to suit a bond with the substrate. This is non to suggest that an enzyme'due south active site is completely malleable, however. Both the lock-and-cardinal model and the induced fit model account for the fact that enzymes can only bind with specific substrates, since in general a particular enzyme but catalyzes a particular reaction (Effigy viii).

The Regal Club of Chemical science provides an splendid introduction to enzymes for students and teachers.
Cardinal Concepts and Summary
Catalysts bear upon the charge per unit of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts tin can be homogenous (in the same phase as the reactants) or heterogeneous (a unlike phase than the reactants).
Chemistry Cease of Chapter Exercises
- Account for the increment in reaction rate brought about by a catalyst.
- Compare the functions of homogeneous and heterogeneous catalysts.
- Consider this scenario and answer the following questions: Chlorine atoms resulting from decomposition of chlorofluoromethanes, such every bit CCltwoF2, catalyze the decomposition of ozone in the temper. Ane simplified machinery for the decomposition is:
[latex]\text{O}_3\;{\xrightarrow{\text{sunlight}}}\;\text{O}_2\;+\;\text{O} \\[0.5em] \text{O}_3\;+\;\text{Cl}\;{\longrightarrow}\;\text{O}_2\;+\;\text{ClO} \\[0.5em] \text{ClO}\;+\;\text{O}\;{\longrightarrow}\;\text{Cl}\;+\;\text{O}_2[/latex](a) Explain why chlorine atoms are catalysts in the gas-phase transformation:
[latex]2\text{O}_3\;{\longrightarrow}\;3\text{O}_2[/latex]
(b) Nitric oxide is as well involved in the decomposition of ozone by the mechanism:
[latex]\text{O}_3\;{\xrightarrow{\text{sunlight}}}\;\text{O}_2\;+\;\text{O} \\[0.5em] \text{O}_3\;+\;\text{NO}\;{\longrightarrow}\;\text{NO}_2\;+\;\text{O}_2 \\[0.5em] \text{NO}_2\;+\;\text{O}\;{\longrightarrow}\;\text{NO}\;+\;\text{O}_2[/latex]
Is NO a goad for the decomposition? Explain your answer.
- For each of the following pairs of reaction diagrams, identify which of the pair is catalyzed:
(a)
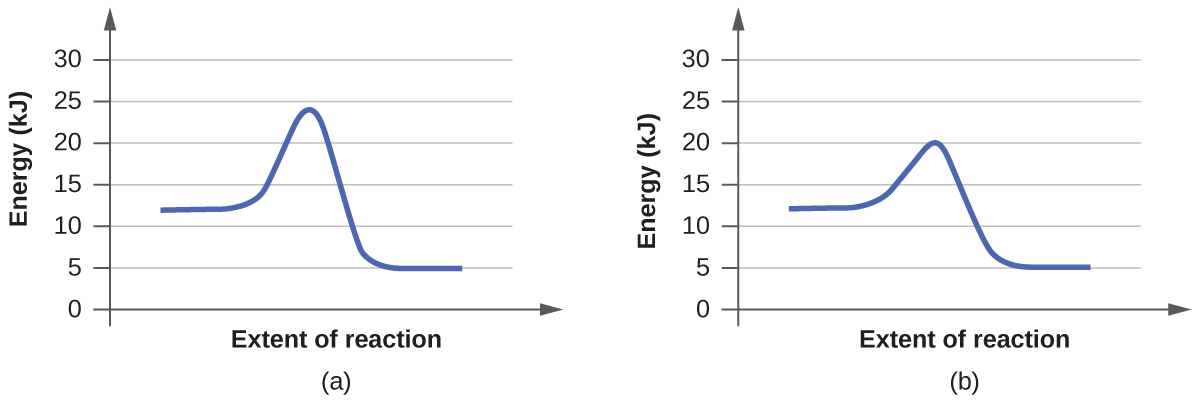
(b)
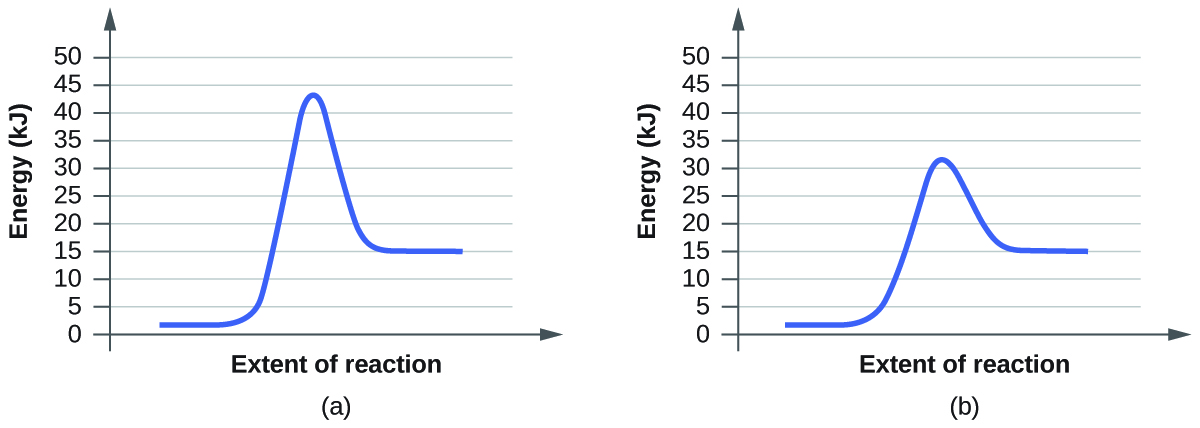
- For each of the following pairs of reaction diagrams, identify which of the pairs is catalyzed:
(a)
(b)
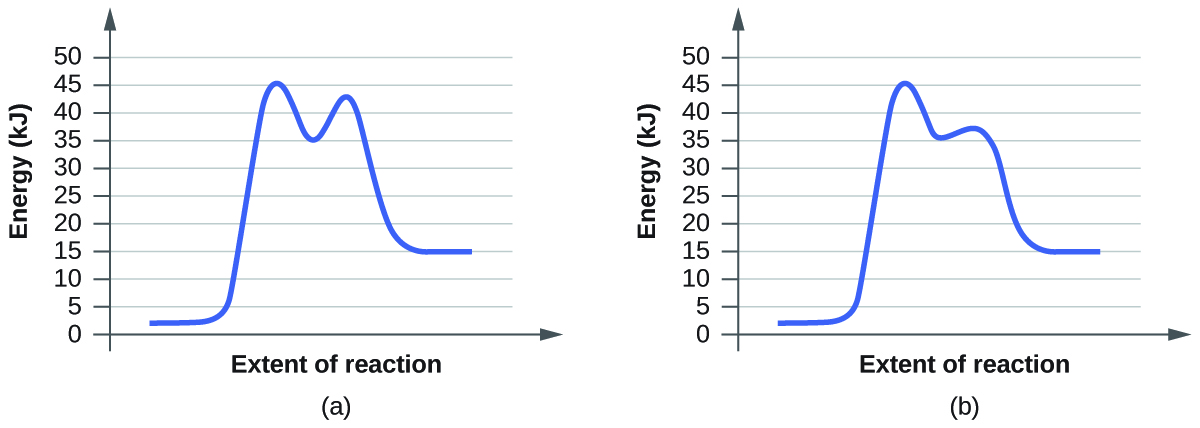
- For each of the post-obit reaction diagrams, judge the activation free energy (E a) of the reaction:
(a)
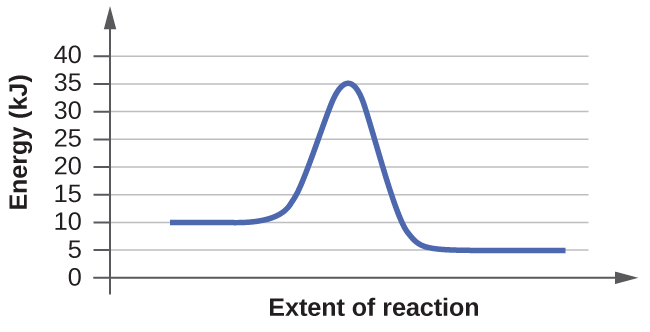
(b)
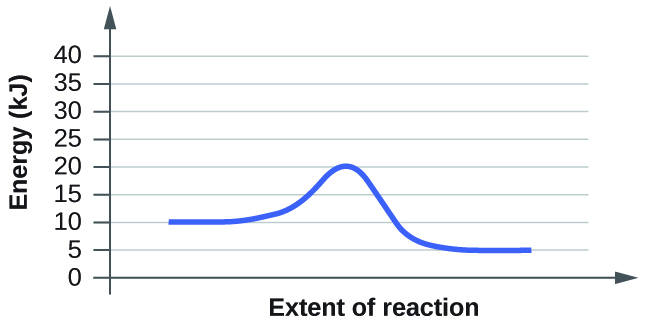
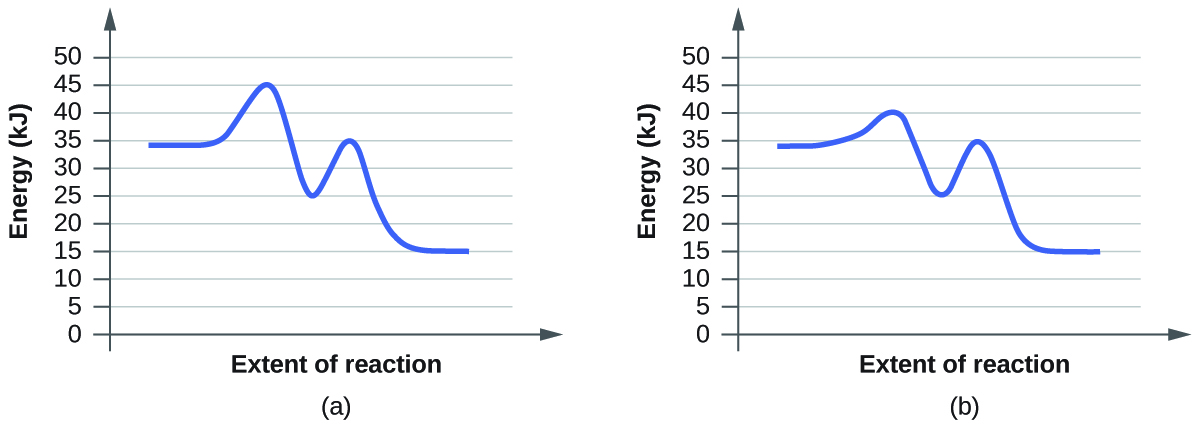
- For each of the following reaction diagrams, judge the activation energy (East a) of the reaction:
(a)
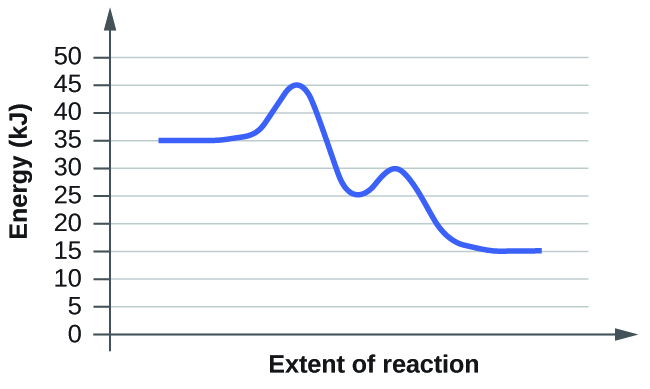
(b)
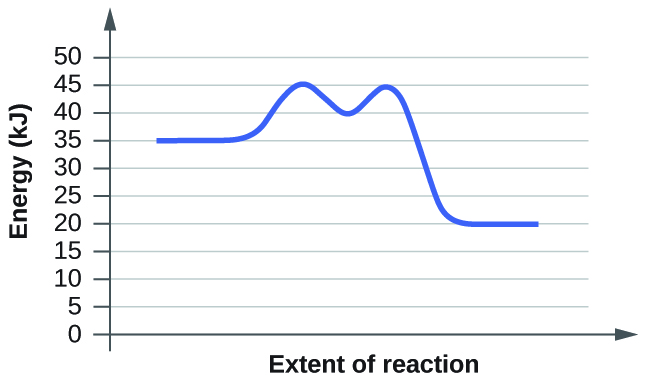
- Based on the diagrams in Chemistry End of Chapter Exercise 6, which of the reactions has the fastest rate? Which has the slowest rate?
- Based on the diagrams in Chemistry End of Chapter Exercise seven, which of the reactions has the fastest rate? Which has the slowest rate?
Glossary
- heterogeneous catalyst
- catalyst present in a different phase from the reactants, furnishing a surface at which a reaction tin can occur
- homogeneous catalyst
- catalyst nowadays in the aforementioned phase as the reactants
Solutions
Answers to Chemistry End of Chapter Exercises
1. The full general way of action for a catalyst is to provide a mechanism by which the reactants can unite more readily past taking a path with a lower reaction energy. The rates of both the forward and the contrary reactions are increased, leading to a faster achievement of equilibrium.
3. (a) Chlorine atoms are a catalyst because they react in the second step just are regenerated in the tertiary step. Thus, they are not used upwardly, which is a characteristic of catalysts. (b) NO is a goad for the aforementioned reason as in part (a).
5. The lowering of the transition country free energy indicates the effect of a catalyst. (a) A; (b) B
7. The energy needed to go from the initial land to the transition state is (a) 10 kJ; (b) 10 kJ
ix. Both have the same activation free energy, and so they as well have the same charge per unit.
Which Two Substances Bind Using A Lock-and-key Mechanism?,
Source: https://opentextbc.ca/chemistry/chapter/12-7-catalysis/
Posted by: maurinjoyesugly.blogspot.com


0 Response to "Which Two Substances Bind Using A Lock-and-key Mechanism?"
Post a Comment